This is an archived article that was published on sltrib.com in 2014, and information in the article may be outdated. It is provided only for personal research purposes and may not be reprinted.
Utah doctors and hospitals raked in more than $31.2 million from medical device makers and drug companies at the end of 2013.
Just a fraction of their names and payment amounts appear on the government's new transparency website, Open Payments.
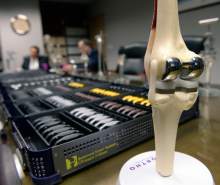
The data where doctors are identified, however, tell an interesting story about Utah's medical profession: Some orthopedic surgeons make a tidy sum working with industry to invent and improve surgical instruments and artificial hips, knees and other implants.
Nearly two-thirds of the $4.8 million in payments to Utah doctors identified by name on the new transparency website were for royalties and licenses, mostly in orthopedics and sports medicine. Only 9 percent was for wining, dining and travel.
That may explain surgeon Marc Mariani's fear that the data, public for the first time as a provision of the Affordable Care Act, will be misunderstood.
"If you just look at the number, people will say, 'Oh those greedy guys,'" says Mariani, who earned $298,749 over the five months, nearly all of it royalties paid by Ortho Development Corp. of Draper.
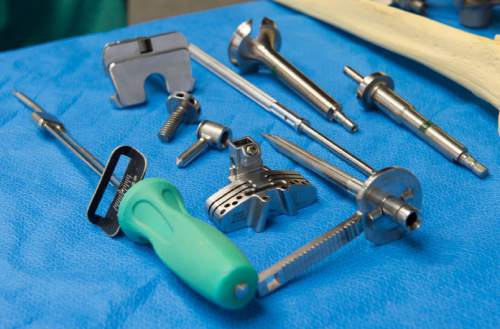
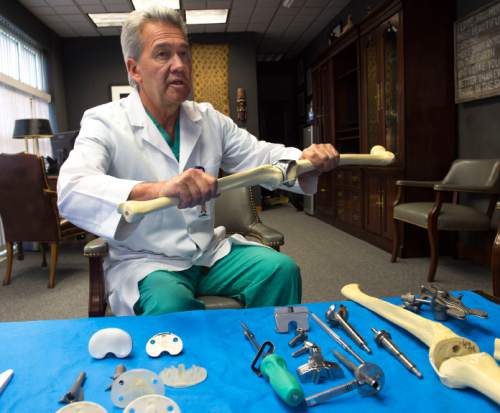
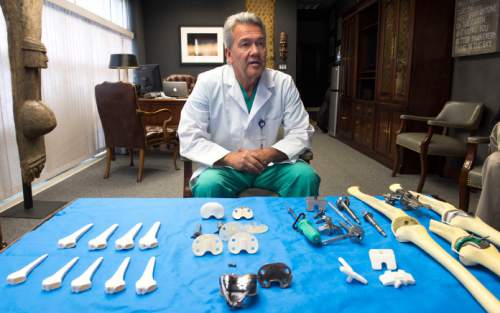
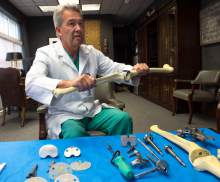
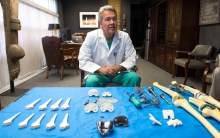
He and Michael Bourne co-founded The Salt Lake Orthopaedic Clinic, housed at St. Mark's Hospital, and have been working with the Draper company to invent and refine knee and hip implants and surgical equipment since the late 1990s.
The two orthopedic surgeons ranked No. 4 (Bourne) and No. 5 (Mariani) on the list of doctors receiving the most money from industry, according to an analysis of the data with identified recipients.
"What you're seeing is the end result of 16 years of hard work," says Mariani.
Bourne, who earned $308,610 from his relationship with Ortho Development, says collaboration between surgeons and industry is at the heart of innovation in medicine.
"An engineer can put things together, but that doesn't mean you can implant it," says Bourne. "You have to have surgeons involved."
Tom Calton, an orthopedic surgeon in Ogden, is working with Ortho Development now on improvements to an existing device. But, he says, "It's years away from going to market."
Calton was No. 9, with $99,194, all from Ortho Development and almost all royalties on instruments he helped invent to get more precision in knee implant surgeries.
Ortho Development's reading of federal anti-kickback laws has the company put in every contract — Mariani, Bourne and Calton's included — that a physician can make no royalty off any implants he uses or his partners use.
"It's cleaner and it's the right thing to do," says Mariani. "I never want somebody to think I'm treating them or using a product because I'm getting a royalty from it."
—
Paid on the side • It was industry's scandalous involvement with doctors that led to the transparency mandate in the first place.
Pharmaceutical companies paid billions of dollars in fines over the past decade to avoid criminal and civil charges for illegally compensating doctors for prescribing their drugs.
And in 2007, four big orthopedic device makers paid $311 million to settle federal charges that they illegally paid surgeons — via lucrative consulting agreements and other inducements — for using their artificial knees and hips. A fifth orthopedic company later reached a settlement of similar charges.
Conflict-of-interest policies were adopted by professional medical groups, universities and medical journals, and now the Centers for Medicare & Medicaid (CMS) in the U.S. Department of Health and Human Services has taken its first stab at making industry-doctor relationships transparent: the Open Payments program.
Mandated by the Congress in the Physician Payments Sunshine Act (part of Obamacare), Open Payments requires drug and device makers to report payments of more than $10 to doctors and teaching hospitals.
That means that every box of doughnuts delivered by a drug rep to a doctor's office must be reported to the feds, as must every honorarium for speaking or teaching (and the travel involved), every consulting fee, every royalty for intellectual property, each research grant and every reimbursement for running clinical trials.
The first set of data, posted by CMS on Sept. 30, covers the last five months of 2013. Data for 2014 are to be posted next June.
The detail presented so far, however, is far from complete.
The CMS says it was unable to verify 40 percent of the payments reported by companies so there are no physician or hospital names attached to more than 1.7 million of the 4.4 million payments for the five months.
In total, companies said they paid 546,000 physicians and 1,360 teaching hospitals $3.5 billion during the period. (This doesn't include ongoing research that companies can shield for a time.)
An analysis of the payments in which the physician or hospital is named reveals that 1,500 Utah doctors received nearly $4.8 million, the University of Utah got more than $3 million and four other Utah hospitals got $68,000.
Companies reported paying much more to Utah doctors and hospitals that are unidentified — for now, nearly $18.7 million for research and $5.1 million in general payments. The CMS said it expects to update the data next year, as it verifies who got what.
—
Website glitches • Critics are drawing parallels between Open Payments and the early days of healthcare.gov last fall. The same contractor built both websites.
"As a casual observer, it's pretty esoteric and complicated," says Brian Watts, chief compliance officer for University of Utah Health Sciences.
It took several weeks this fall for CMS to debut a consumer-friendly tool on its website so patients can quickly search their own doctor's payments.
To drill down to learn more, a user must go to spreadsheets at the site's Data Explorer link.
The American Medical Association criticizes Open Payments for not allowing doctors more time and an easier way to scrutinize the companies' reports before the data went public. They had several weeks over the summer, but the website was down part of the time.
Robert Cionni, an ophthalmologist and founder of The Eye Institute of Utah, says he gave up trying because, "It was a nightmare."
Between his travel, speaking schedule and cataract removal surgeries, he decided not to even try sorting out whether the companies got it right.
Cionni was the only Utah physician to fall near the top of both kinds of payments — for research and for general payments such as consulting and speaking fees.
Alcon paid Cionni $98,930 to run clinical trials on new cataract surgery methods, and the company accounted for most of the $80,730 he made as a consultant and speaker.
Patients should know, he says, that's not all profit. For instance, the Eye Institute employs four full-time staffers who help run clinical trials, and Alcon's reimbursements help cover that expense. Speaker fees and travel costs typically do not make up for lost time in surgery, he says.
Charles Beck, an orthopedic surgeon in West Jordan, says that while there were some egregious examples of graft between industry and surgeons, the new transparency push is "overkill."
"It's assuming everybody is a criminal," says Beck, who works at the Center for Orthopedic and Rehabilitation Excellence based at Jordan Valley Medical Center.
He did not even try to verify the more than $213,000 in royalty payments DePuy Mitek, a big orthopedic manufacturer, reported paying him. He ranked No. 6 in the state.
He invented a device that surgeons around the world use to attach ligament to bone during knee ligament reconstructions, getting, he says, about $1 or $2 per device.
Beck says he uses the device in only 75 of his 700 surgeries each year.
Greg Larson, Ortho Development's chief financial officer, says it took him a day and a half to navigate the site and submit to a background check so he could report the company's payments.
And he's mystified that the CMS couldn't verify some of Ortho's data and thus did not report the names of those getting some payments.
Nonetheless, he said, "Transparency is good, if you can get rid of any improprieties."
Watts at the U. hopes Open Payments will ultimately provide the context patients need for clarity about their doctor's or hospital's relationship with industry.
Open Payments, for instance, reports the university got dozens of research payments totaling nearly $2.3 million. But one learns the names of the principal researchers and their projects only by drilling down through spreadsheets.
Nassir Marrouche, a U. cardiologist who landed at No. 10 on the list of doctors receiving money from industry, is also listed as the principal investigator on one of the U. research payments, for $10,000 from eCardio Diagnostics.
According to Open Payments, Marrouche got $35,500 from eCardio, mostly for consulting, and a $51,430 honorarium from Biotronik Inc., by far the biggest honorarium for any Utah doctor.
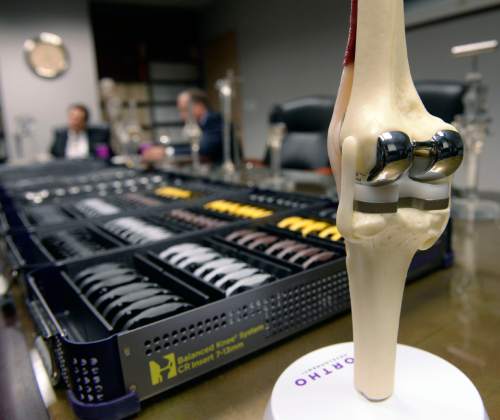
G. Lynn Rasmussen, an orthopedic surgeon and president of the medical staff at Intermountain Healthcare's The Orthopedic Specialty Hospital, ranked No. 3 among the doctors getting the most from industry late last year.
But some of $393,949 he was paid, he says, was royalties that had been held up for years as the Justice Department investigated Smith & Nephew Inc., the orthopedic company that sells the knee implant he patented.
The royalties finally came through all at once last fall, artificially inflating what he would normally receive in a five-month span.
Rasmussen also got a one-time licensing fee from Wright Medical Technology Inc. for surgical instruments he developed to better balance knee implants.
He notes he does not get royalties for any device he or others at TOSH use. And that's a good way to keep conflict-of-interest at bay, he says.
Requiring industry to sever all ties with surgeons, he says, would be foolhardy. "Even though there are some really bright engineers that work with these companies, they're not the ones in the operating room, using the implant, looking at the results."
—
Long-term benefits • For the patients of Utah doctors collecting income from industry, good outcomes may be more important than public disclosure.
Dawn Messick is in middle-age but already has two new knees and a new hip.
She was in Bourne's office recently for a five-year checkup on the hip, and said she's all for transparency — and for doctors getting a cut of the profits from what they invent.
"They should be rewarded for their innovations because we benefit," says Messick.
"I don't walk with a limp. I don't hurt. I sleep at night. I walk 5Ks. I play with my grandkids," says Messick. "I have a new life."
Others believe the new website will keep doctors and the companies that pay them honest.
It's true that some industry-doctor relationships are valid, says Michael Carome, a physician and director of Public Citizens' Health Research Group. New drugs and devices have to be tested in clinical trials conducted by doctors with real patients, for instance.
But, he says, much of the $3.5 billion that industry paid in five months to doctors and hospitals was all about trying to influence prescribing and device purchasing decisions, he says.
"A good chunk … is corrupting the practice of medicine," says Carome.
Open Payments, he says, has the potential to shed light on what's been "hidden and occurring behind the scenes," he said.
"It will only improve with time."
Twitter: @KristenMoulton —
On the Web
O To see the open payments website, go to > http://1.usa.gov/1ovAE78