This is an archived article that was published on sltrib.com in 2012, and information in the article may be outdated. It is provided only for personal research purposes and may not be reprinted.
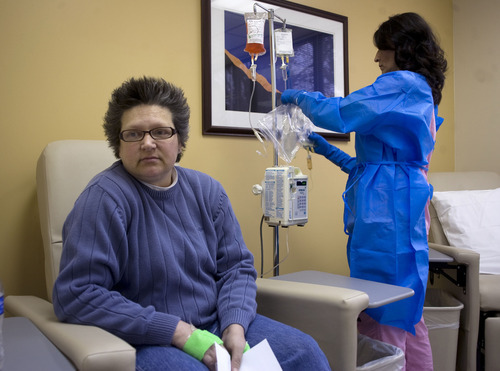
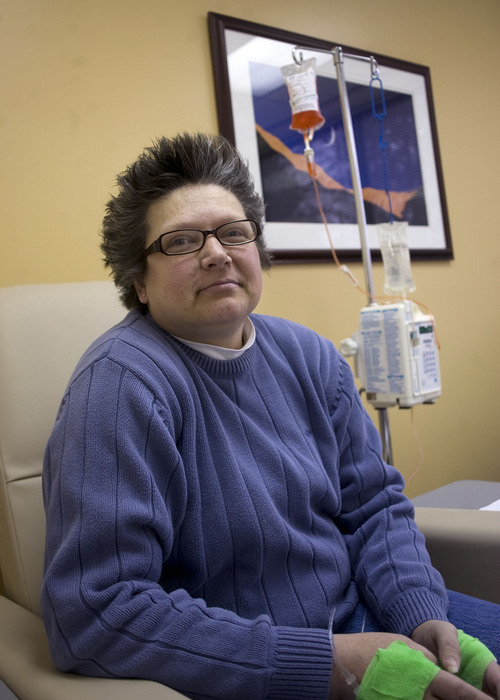
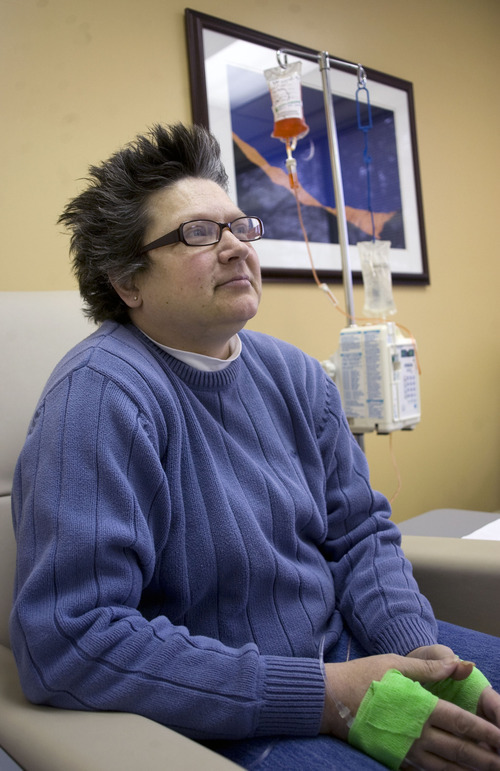
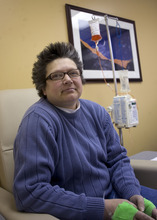
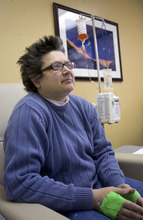
Morning sun peeks through the blinds of an infusion room as Brenda Olson settles in for a two-hour intravenous dose of Doxil.
The chemotherapy drug won't cure her ovarian cancer, but it's keeping the cancer's growth in check and prolonging her life — for now.
This is dose two of four allotted to her under a rationing program run by the drugmaker Janssen. "We don't know if I'll be able to get more," said the 44-year-old West Jordan woman.
Collaborative efforts by drugmakers and federal regulators to boost supplies of life-saving medicines — particularly cancer drugs — are helping to ease a nationwide shortage, say doctors and pharmacists. But the fixes are temporary and limited in scope, with no permanent relief in sight, they say.
"It's a good start. But we're still sweating shortages every single day," said Erin Fox, manager of the Drug Information Service at the University of Utah, a national tracking system that alerts the Food and Drug Administration (FDA) to shortages.
There have been 27 new shortages this year, down from 60 by this time in 2011, said Fox. But 283 drugs remain in short supply, exceeding last year's record of 267, she said.
Health providers are running low — or completely out — of cancer therapies and staples such as antibiotics and injectable drugs used for sedation and in emergencies to treat strokes, heart attacks and allergic reactions.
At fault are federal recalls, manufacturing failures and corporate decisions to discontinue less profitable medicines.
In October, President Barack Obama signed an executive order requiring drug companies to notify the FDA of manufacturing disruptions, which the administration says has prevented 195 shortages. In addition, the FDA took the extraordinary step last month of allowing the foreign importation of a Doxil "replacement" drug, Lipodex.
And it approved a new supplier for a preservative-free form of methotrexate, which is crucial for children with acute lymphocytic leukemia and used to treat the bone cancer osteosarcoma.
"Patients and families waiting for these products or anxious about their availability should now be able to get the medication they need," said FDA Commissioner Margaret Hamburg in a statement.
Fox applauds the moves. The methotrexate shortage was an especially deadly one because the drug is so widely used, she said.
She has no quality concerns with Lipodex, which is made by Sun Pharma Global in India. "We use drugs from one of their subsidiaries," Fox said. "The FDA has to make sure it's safe, and there's enough of a supply so as not to create a shortage elsewhere."
But doctors say it's unclear how interchangeable Lipodox and Doxil are, how much Lipodox will be available and for how long.
"I've never used the drug," said Richard Frame, the oncologist treating Olson. "We don't even know how much to charge."
Prescribing pamphlets recommend Doxil for recurrent ovarian cancer, AIDS-related Karposi's sarcoma and multiple myeloma. Lipodox can be prescribed for all but multiple myeloma and works against breast cancer.
When Olson started on Doxil "there was no hint" that the supply would dwindle, said Frame, a member of the community oncology group Utah Cancer Specialists.
A carrier of the BRCA1 gene, Olson beat breast cancer in the 1990s and was diagnosed with stage III ovarian cancer in 2006.
She underwent surgery and tried three different chemotherapy drugs. But by 2009 she had developed a large tumor in her lower abdomen, and the cancer had spread to her lymph nodes.
When radiation didn't work, she says, "I stopped discussing or making long-term plans."
Then, in March 2011, Frame suggested she try Doxil. Olson's tumor shrank, she had almost no pain and few side effects. "I felt almost normal," she says.
But six months ago, she learned the drug was no longer available. "I've never had to tell a patient that before," said Frame.
It took four months to get Olson on the Doxil rationing program, during which time the tumor in her abdomen grew to the size of an orange, and she developed another mass on her spine.
Olson hopes to remain on the drug, but there are no guarantees. "It just saddens me to know others are still waiting and dying," she says.
Frame is angry. Though optimistic that Lipodox will work for his patients, he sees no end to the drug shortage without Congress addressing the underlying problem of drug pricing.
"When these drugs are no longer on patent, there is a race to the bottom to see who can produce the drug at the cheapest cost," Frame said. "This weeds out a lot of pharmaceutical companies who say, 'There's no profit, no monetary reason to produce these drugs.' "
Doxil is unique in that it's not a generic medication. In an unusual move, Janssen, a division of Johnson & Johnson, picked one facility to produce the world's supply of the drug, Ben Venue Laboratories in Bedford, Ohio.
Janssen has blamed the shortage on equipment failure at Ben Venue and is looking for another supplier.
But multiple facilities are in serious disrepair, said Fox, citing FDA reports of mold and rusty metal shavings falling into IV solutions. "This problem won't go away until the drugmakers fix their factories."
kstewart@sltrib.comTwitter: @kirstendstewart