This is an archived article that was published on sltrib.com in 2012, and information in the article may be outdated. It is provided only for personal research purposes and may not be reprinted.
Bonnie Moncur's doctor recommended an MRI just to be safe.
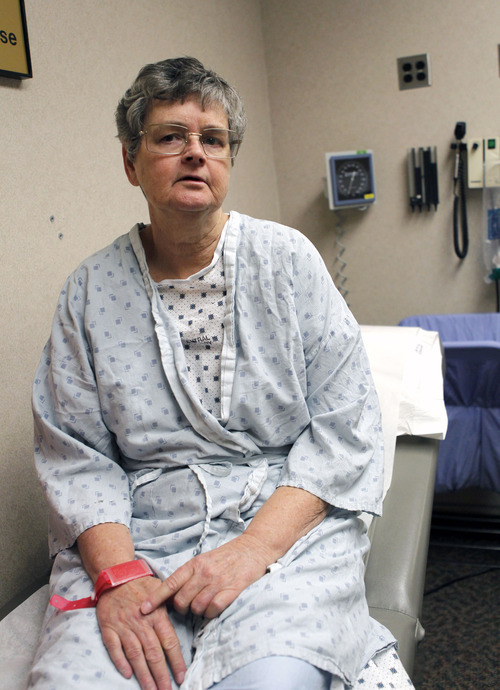
The 61-year-old Davis County woman was having frequent headaches and her younger sister had just died from a ruptured brain aneurysm that no one knew about until it was too late. Moncur's scan revealed a 4-millimeter bulge in an artery in her brain — terrible knowledge that she lived with for two years, wagering the risks of surgery were greater than the risks of rupture.
But the watchful waiting is over. Two weeks ago Moncur became the seventh patient at the University of Utah to benefit from a minimally invasive treatment approved in 2011 by the Food and Drug Administration: a "Pipeline Embolization Device," or a stent, placed inside an artery across the bulging aneurysm, inserted trough a catheter threaded up through the thigh.
The Pipeline device has been used in hundreds of patients at dozens of health centers across the country, but is new to Utah.
"It's a real paradigm shift. People travel all around the world for this procedure," said U. neurosurgeon Philipp Taussky, believed to be the only doctor in the Intermountain West trained to use it.
Taussky was hired away from the Mayo Clinic, in part, to bring the technology here. He did not have a role in its creation by the Massachusetts-based device maker, Covidien, and has no financial stake in it, but while at the U. he will participate in a clinical trial to test the next generation of Pipeline.
Cerebral aneurysms, a ballooning or weakness in the arterial wall, occur in about 2 percent of the population, according to the Brain Aneurysm Foundation. Ruptures are fatal about 40 percent of the time. Most of those who survive, about 66 percent, are permanently disabled, the foundation says.
One potential treatment is open surgery, which requires cracking open the skull and clipping off the aneurysm. Such procedures can take 18 hours to perform and require weeks, or months, of recovery, said Taussky.
Doctors at Intermountain Medical Center in Murray also treat aneurysms with catheter-placed stents in combination with coiling — or packing the aneurysm with soft platinum coils. Blood will clot around the coils, blocking blood flow to the aneurysm wall. The stent keeps the coils from slipping.
But the advantage of Pipeline, said Taussky, is that rather than blocking the flow of blood to the bulge, it diverts it. In three to six months, the aneurysm generally shrinks and grows around the stent's tightly woven mesh, he said. "The stent becomes part of the vessel wall."
The device is recommended for larger aneurysms of 5 or more millimeters in diameter.
It's not without complications.
A study by the National Institutes of Health pegged the risk of death at 5 percent, about the same as with coiling.
That compares to a mortality rate of 25 percent with open surgery.
But, in Moncur's case, the device may have spared her some loss of sight.
"Coming off her aneurysm was an artery feeding her right eye. Had we coiled that, it would have occluded the artery," blocking blood flow, he said.
It took Taussky 60 minutes to place the stent. Two days later, Moncur was home, still sick to her stomach from the anesthesia, but able to resume normal activity. "I don't feel any pain," she said.
Her aneurysm was only 4 centimeters wide, but Taussky said her sister's demise increased her risk of rupture sevenfold.
"If they would have had to open me up, I wouldn't have gotten it fixed," she said." But I'm glad I did. It's a relief."