This is an archived article that was published on sltrib.com in 2014, and information in the article may be outdated. It is provided only for personal research purposes and may not be reprinted.
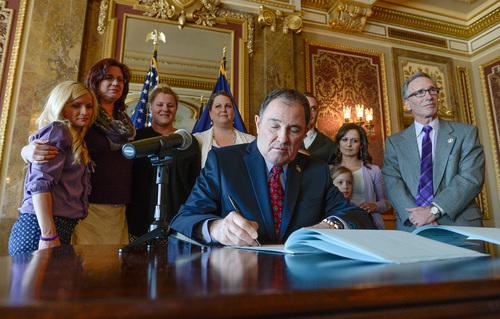
Utah's hemp supplement bill, better known as "Charlee's Law," is a technical piece of legislation, packed with a lot of "legalese," said sponsor Rep. Gage Froerer at the governor's ceremonial signing of the law on Tuesday.
But it's a symbol of compassion for parents who have exhausted all medical remedies for their children, and a promise of hope for a better future, he said.
"And number one it's about trust," Froerer said. "The state of Utah is willing to trust the parents of this state in making the decisions they feel are right for their children's health."
HB105 gives Utahns with epilepsy trial access to a non-intoxicating, seizure-stopping cannabis oil. But it doesn't take effect until July 1, 2014, and until then, Utahns can't legally possess cannabis oil.
And obtaining it after that date will still risk violating federal law — and require jumping through a set of still-vaguely defined hoops.
Currently, patients will need to travel to states where medical marijuana is legal and import cannabis oil themselves. Doing so remains technically a violation of federal law.
That hasn't stopped states from enacting their own marijuana laws.
Utah Gov. Gary Herbert signed HB105 last week. At Tuesday's ceremonial signing at the Capitol he applauded families for being the driving force behind a bill that many surmised had no chance of passage and that underwent nine revisions.
Herbert initially had quality control and safety concerns, but said, "I'm satisfied ... that we have a bill that is where it needs to be."
At the event Tuesday were grassroots organizers of Hope 4 Children With Epilepsy (H4CE) wearing purple in honor of March 26, which is Epilepsy Awareness Day. The parents of the bill's namesake, Charlee Nelson, one of 50 Utah children on a Colorado waiting list for nonintoxicating cannabis oil, were there. The 6-year-old West Jordan girl died two weekends ago, days after being honored by the Legislature.
Still numb and processing her daughter's death, Catrina Nelson lamented that Charlee "couldn't be here with us today." But she said, "I'm excited to see this work out for so many other families."
It will be months before the Utah Department of Health fine tunes the process for families to apply for a "hemp supplement" registration card.
The law requires families to first obtain a letter of recommendation from their neurologist, and hospitals are bracing for an influx of calls from families eager to get the ball rolling.
"We are committed to working with these families. We want to be their partner in this journey," said Edward Clark, pediatrics chairman at University of Utah Health Care and chief medical officer at Intermountain Healthcare's Primary Children's Hospital.
But until the health department issues rules, hospitals can't provide much guidance to patients.
Neurologists are in short supply and high demand in Utah and most of those specializing in untreatable epilepsy work at the U. and at Primary Children's.
It's unclear how many adults and children in Utah are candidates for cannabis oil, but most probably already have records on file at these hospitals, said Clark.
He has assigned a task force to create a pipeline to speed requests for recommendation letters. The U. will likely sponsor a hotline where patients can register for information, he said.
"Some patients may require additional testing and some may not," he said. "I've asked our epileptologists to define what they need. ...There are people working on this every day trying to get these pieces assembled."
The U.'s doctors hope to study cannabis oil and chart patients' progress on it. On a parallel track they have also applied to run an investigational trial of a pharmaceutical-grade cannabis extract for use in children with a severe form of epilepsy called Dravet syndrome.
The drug, Epidiolex, is a liquid, purified form of cannabidiol (CBD), a non-psychoactive component of marijuana, made by British drug maker GW Pharmaceuticals.
Clark couldn't say when the trial might start or how many patients might be eligible. It may be possible to design the study to avoid having to give placebos to children, a common concern of parents, he said. "It could be an open label trial."
All this, however, hinges on the availability of funding and approval by GW and the U.S. Food and Drug Administration and Drug Enforcement Administration, Clark said.
Additionally, the U. has applied to the National Institutes of Health to become part of a national Undiagnosed Disease Network: academic medical centers handpicked to provide answers to patients with rare, mysterious conditions that elude diagnosis. Uncontrolled seizures are often the byproduct of these so-called orphan diseases.
All these moving parts present challenges for families trying to do best by their children.
But Jennifer May, the Utah County mother of a boy with Dravet syndrome and co-founder of H4CE, says she's thrilled to have choices.
"No child responds to the same treatment. We need every option available," she said. There are about 45 anti-epileptic drugs on the market and most children try 20 or more before finding a few that work, or exhausting all medical remedies, she said.
Some may decide to sign up for the Epidiolex trial first, since the drug's maker may not accept patients taking artisanal oils made by marijuana growers, May said.
But others, like Annette Maughan, president of the Epilepsy Association of Utah, have more comfort with artisanal oils that have been documented to work in two observational trials of 180 children, and shown to have few side effects.
Early results of Epidiolex's trials are not publicly available, she said. Still, she is eager for more testing of more products.
"I want that quality control," she said. "Any validation we can have and any protection we can ensure for our children, great."
Twitter: @KStewart4Trib —
Read more about how the new hemp supplement law will work
Parents' group: How to comply with Utah's new cannabis law