Steve Griffin / The Salt Lake Tribune
Intermountain Medical Center Heart Institute electrophysiologist John Day, MD, left, perfo
Steve Griffin / The Salt Lake Tribune
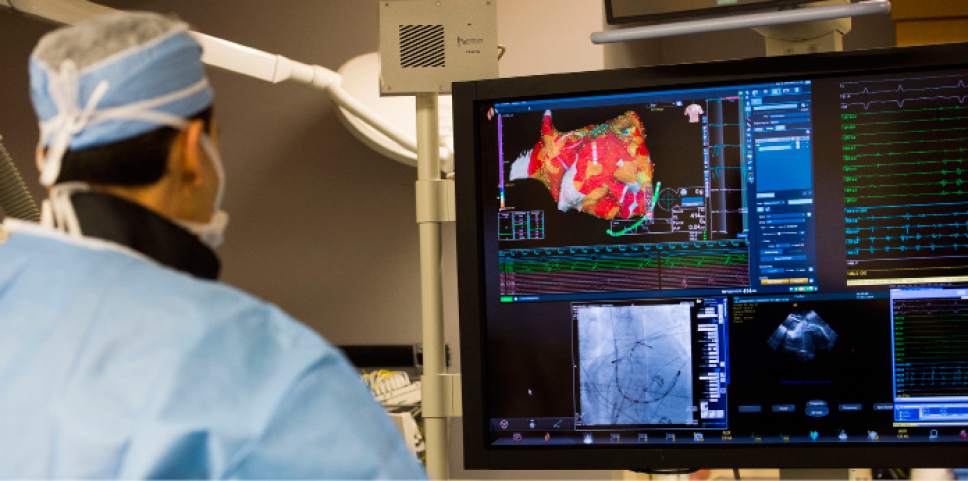
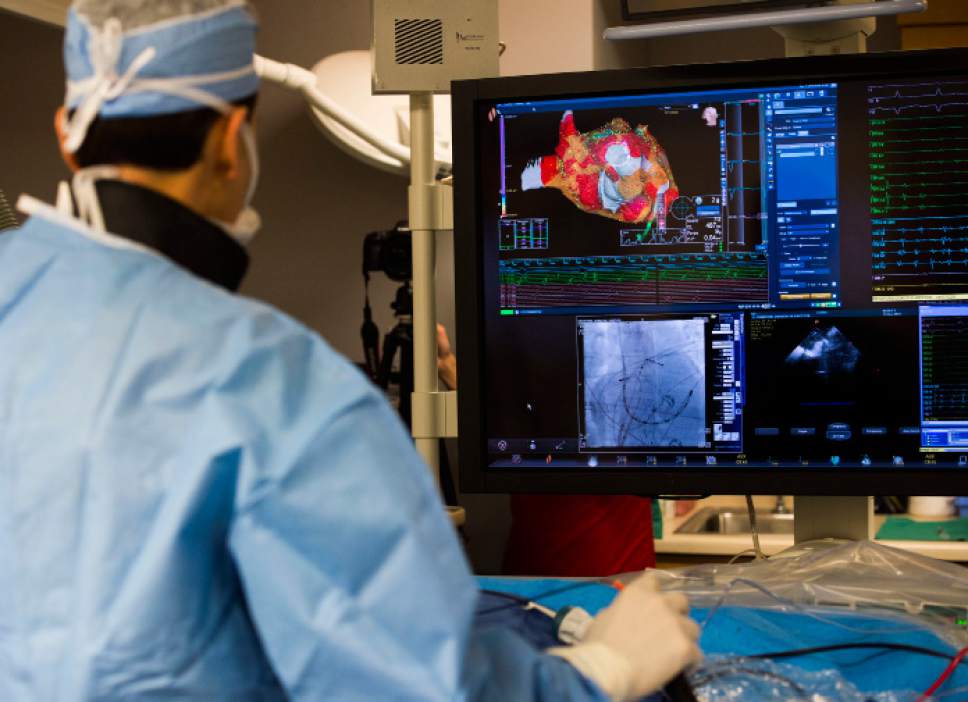
A real-time 3D image of a patient's heart is viewed on a screen as Intermountain Medical
Steve Griffin / The Salt Lake Tribune
Intermountain Medical Center Heart Institute electrophysiologist John Day, MD, left, perfo
Steve Griffin / The Salt Lake Tribune
Intermountain Medical Center Heart Institute electrophysiologist John Day, MD, center back
Steve Griffin / The Salt Lake Tribune
Intermountain Medical Center Heart Institute electrophysiologist John Day, MD, left back,
Steve Griffin / The Salt Lake Tribune
Intermountain Medical Center Heart Institute electrophysiologist John Day, MD, left, perfo
Steve Griffin / The Salt Lake Tribune
Intermountain Medical Center Heart Institute electrophysiologist John Day, MD, left, perfo
Steve Griffin / The Salt Lake Tribune
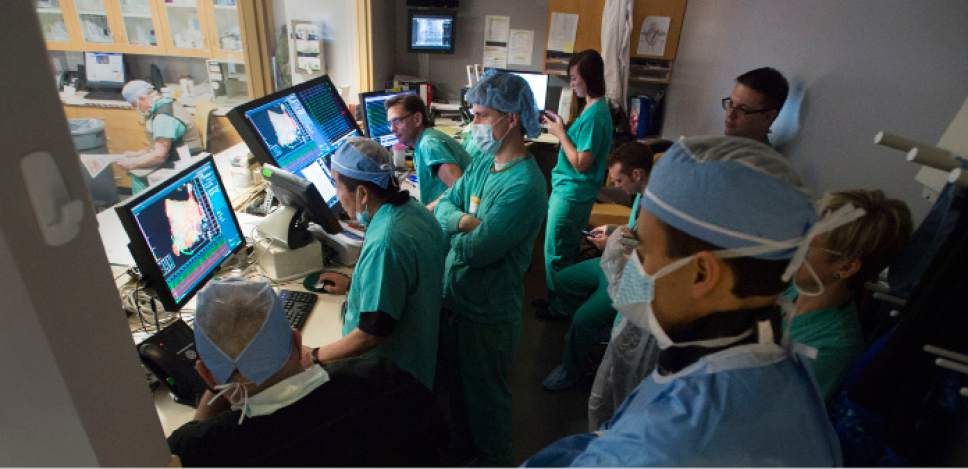
Medical staff watches as Intermountain Medical Center Heart Institute electrophysiologist
Steve Griffin / The Salt Lake Tribune
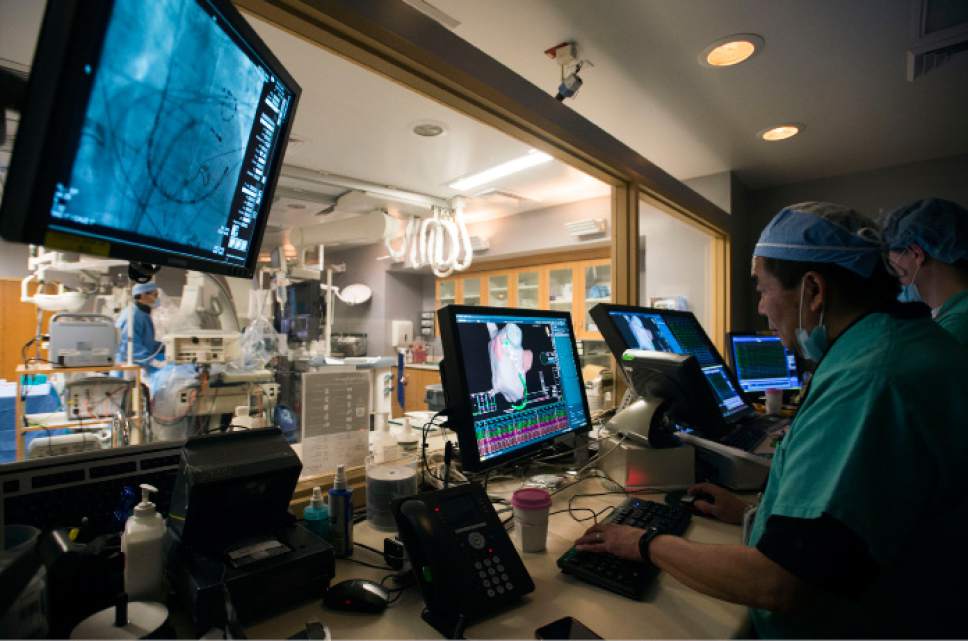
Intermountain Medical Center Heart Institute electrophysiologist John Day, MD, left, performs the nationís first case using new heart-mapping technology for the treatment of abnormal heart rhythms at the Intermountain Medical Center Heart Institute in in Murray Monday December 19, 2016. During the procedure, the Intermountain Medical Center team, used St. Jude Medicalís EnSite PrecisionTM cardiac mapping system to create 3-D images of the heart.
Steve Griffin / The Salt Lake Tribune
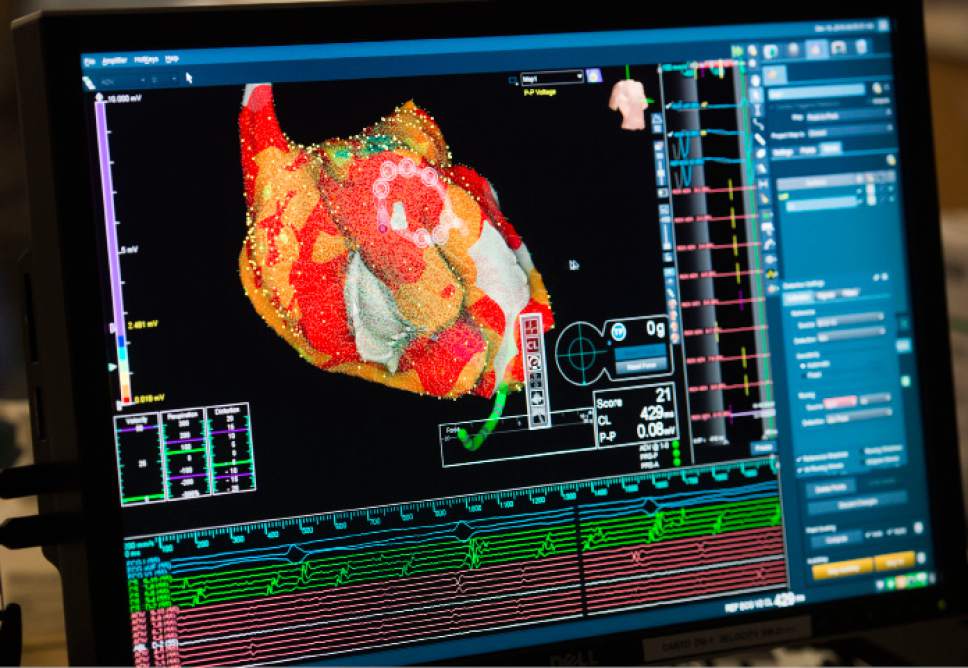
A real-time 3D image of a patient's heart is viewed on a screen as Intermountain Medical Center Heart Institute electrophysiologist John Day, MD, left, performs the nation's first case using new heart-mapping technology for the treatment of abnormal heart rhythms at the Intermountain Medical Center Heart Institute in in Murray Monday December 19, 2016. During the procedure, the Intermountain Medical Center team, used St. Jude Medical's EnSite PrecisionTM cardiac mapping system to create 3-D images of the heart.
Steve Griffin / The Salt Lake Tribune
Intermountain Medical Center Heart Institute electrophysiologist John Day, MD, left, performs the nation's first case using new heart-mapping technology for the treatment of abnormal heart rhythms at the Intermountain Medical Center Heart Institute in in Murray Monday December 19, 2016. During the procedure, the Intermountain Medical Center team, used St. Jude Medical's EnSite PrecisionTM cardiac mapping system to create 3-D images of the heart.
Steve Griffin / The Salt Lake Tribune
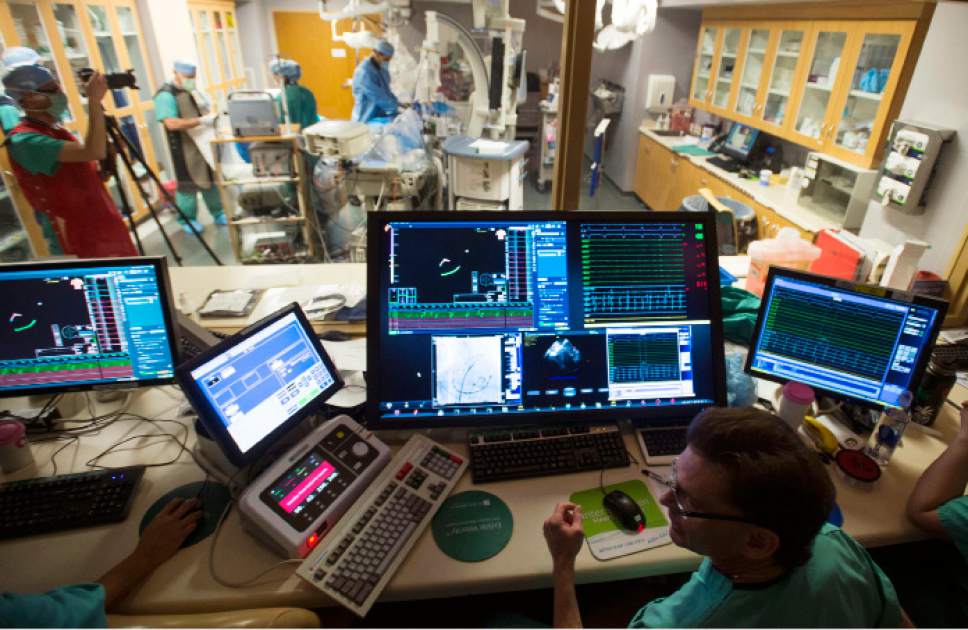
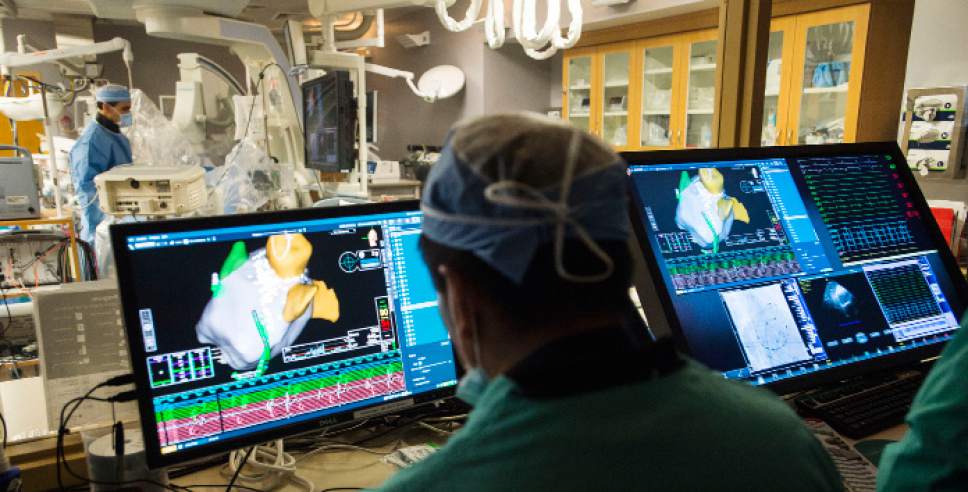
Intermountain Medical Center Heart Institute electrophysiologist John Day, MD, center back, perform the nation's first case using new heart-mapping technology for the treatment of abnormal heart rhythms at the Intermountain Medical Center Heart Institute in in Murray Monday December 19, 2016. During the procedure, the Intermountain Medical Center team, used St. Jude Medical's EnSite PrecisionTM cardiac mapping system to create 3-D images of the heart.
Steve Griffin / The Salt Lake Tribune
Intermountain Medical Center Heart Institute electrophysiologist John Day, MD, left back, performs the nation's first case using new heart-mapping technology for the treatment of abnormal heart rhythms at the Intermountain Medical Center Heart Institute in in Murray Monday December 19, 2016. During the procedure, the Intermountain Medical Center team, used St. Jude Medical's EnSite PrecisionTM cardiac mapping system to create 3-D images of the heart.
Steve Griffin / The Salt Lake Tribune
Intermountain Medical Center Heart Institute electrophysiologist John Day, MD, left, performs the nation's first case using new heart-mapping technology for the treatment of abnormal heart rhythms at the Intermountain Medical Center Heart Institute in in Murray Monday December 19, 2016. During the procedure, the Intermountain Medical Center team, used St. Jude Medical's EnSite PrecisionTM cardiac mapping system to create 3-D images of the heart.
Steve Griffin / The Salt Lake Tribune
Intermountain Medical Center Heart Institute electrophysiologist John Day, MD, left, performs the nationÌs first case using new heart-mapping technology for the treatment of abnormal heart rhythms at the Intermountain Medical Center Heart Institute in in Murray Monday December 19, 2016. During the procedure, the Intermountain Medical Center team, used St. Jude MedicalÌs EnSite PrecisionTM cardiac mapping system to create 3-D images of the heart.
Steve Griffin / The Salt Lake Tribune
Medical staff watches as Intermountain Medical Center Heart Institute electrophysiologist John Day, MD, left, performs the nation's first case using new heart-mapping technology for the treatment of abnormal heart rhythms at the Intermountain Medical Center Heart Institute in in Murray Monday December 19, 2016. During the procedure, the Intermountain Medical Center team, used St. Jude Medical's EnSite PrecisionTM cardiac mapping system to create 3-D images of the heart.